PRIME CAR-T
CAR-T cell therapy
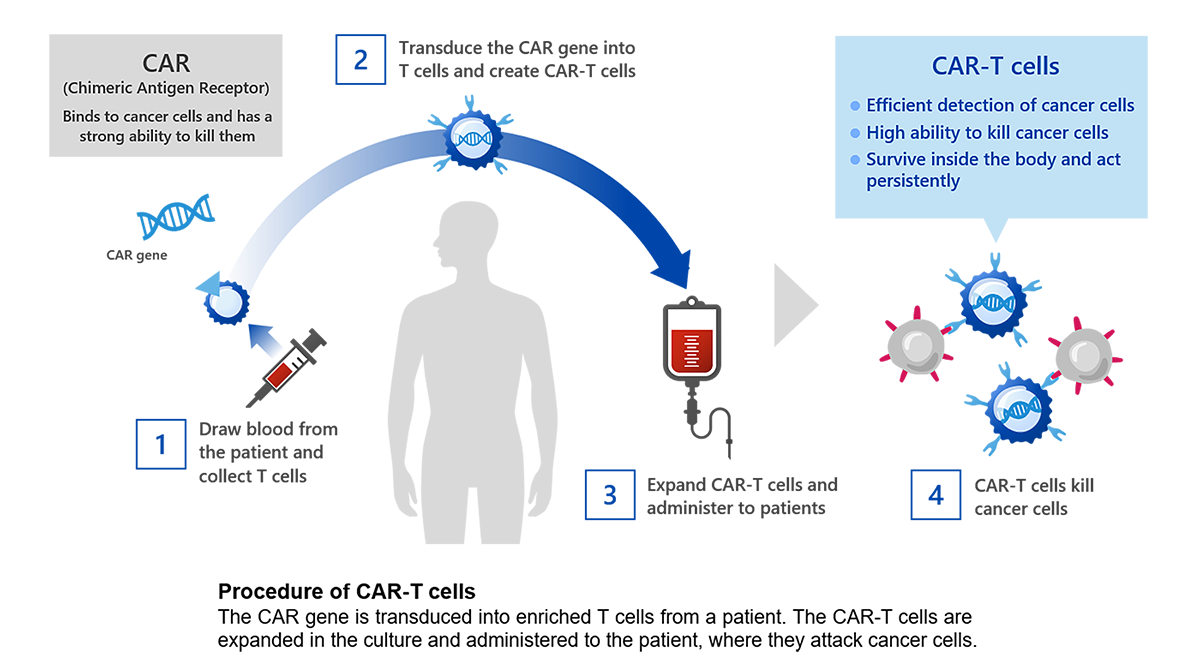
CAR-T cells are manufactured by introducing an artificial gene called a chimeric antigen receptor (CAR) into a type of white blood cell called T cells, which are taken from the patient's blood. The CAR has the ability to detect cancer cells with high sensitivity and to mount a strong attack against them. CAR-T cell therapy is a treatment in which CAR-T cells are expanded in culture for one to two weeks and then administered to the patient. CAR-T cells transfected with the CAR gene recognize and attack the cancer cells that express the target cancer antigen.
Development history and current status of CAR-T cell therapy
CAR-T cell therapy has been developed as a novel therapy for hematological cancers. Most notably, CAR-T cell therapy targeting the CD19 antigen, which is highly expressed in B-cell leukemia/lymphoma, and the BCMA antigen, which is highly expressed in myeloma, has proven effective in clinical trials. As a result, the first CD19 CAR-T cell therapy was approved for these hematological cancers by the US FDA in 2017, the EMA in 2018, and the Japanese authorities in March 2019.
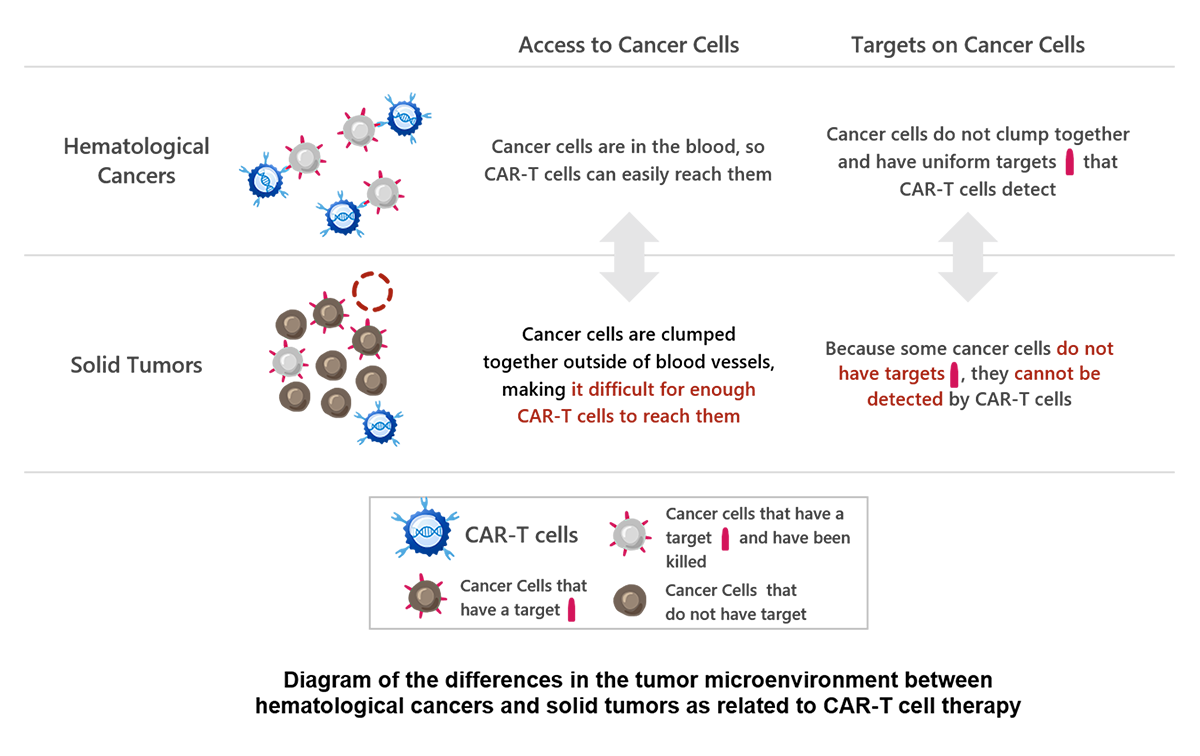
CAR-T cell therapy has been demonstrated to be effective in treatment of hematological cancers; however, it has not been successfully applied to solid tumors. Despite efforts by academic institutions and pharmaceutical companies in many countries to develop CAR-T cell therapies targeting solid tumors, none has been approved to date. One of the known reasons for the difficulty of developing CAR-T for solid tumors is that these tumors differ from blood cancers, as shown in the image below. The delivery of CAR-T cells to localized areas of solid tumors and the heterogeneity of solid tumors (tumor heterogeneity) have been challenges.
Noile-Immune Biotech’s approach
PRIME CAR-T cell therapy
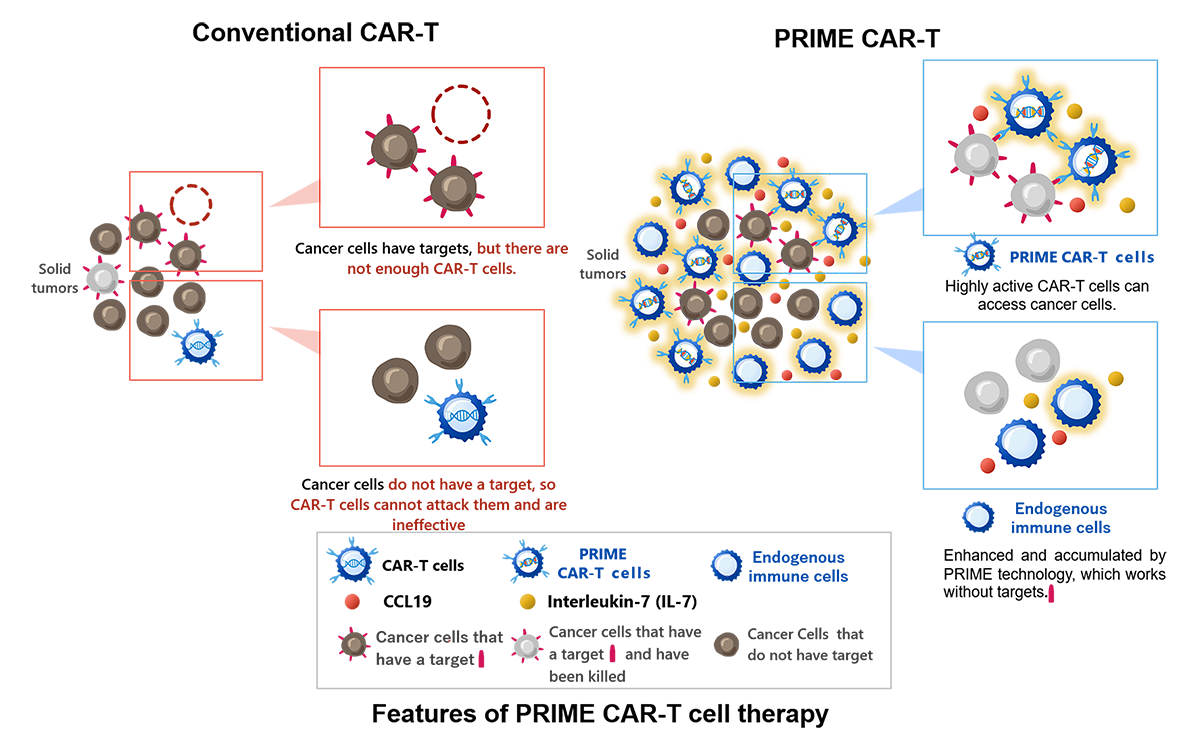
When conventional CAR-T cell therapy is applied, it is difficult to deliver enough CAR-T cells to solid tumor tissues. In addition, although CAR-T cells can selectively attack cancer cells expressing specific cancer antigens, they cannot attack cancer cells that do not express cancer antigens.
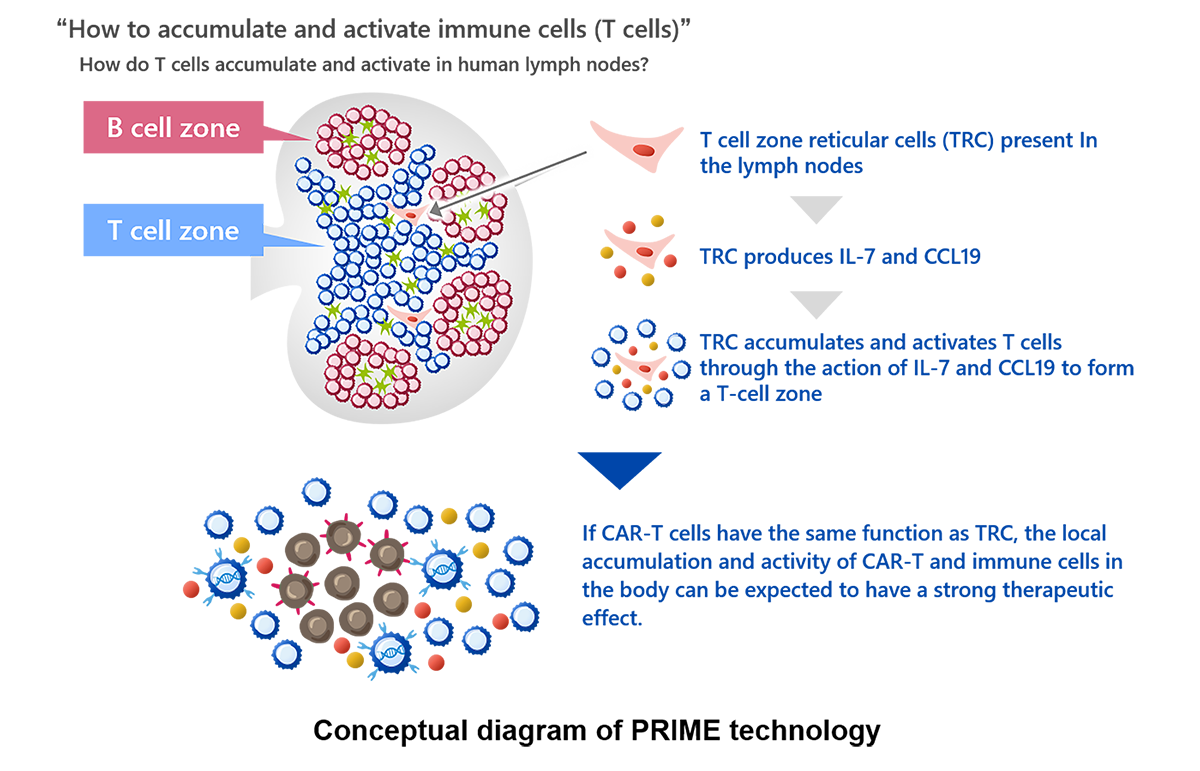
In PRIME CAR-T cell therapy, CAR-T cells are genetically modified to simultaneously produce interleukin-7 (IL-7) and CCL19. IL-7 and CCL19 are immune regulators produced by fibroblastic reticular cells in the T-cell zone of the lymph nodes, where T cells and dendritic cells accumulate. These immune regulators are thought to play crucial roles in the formation of the T-cell zone. IL-7 is known to promote the growth and survival of T cells, and CCL19 is known to enhance the migration of T cells and dendritic cells. PRIME technology was developed to create an environment in which CAR-T cells and the body’s immune cells can easily attack cancer cells by forming a structure at the cancer site that resembles the T-cell zone formed in the body's lymph nodes, as described above.
In mouse models with solid tumors, intravenously administered PRIME CAR-T cells accumulated more efficiently in tumor tissues than conventional CAR-T cells and demonstrated highly potent anti-tumor therapeutic activity (Nature Biotechnology, 36(4):346-351, 2018)
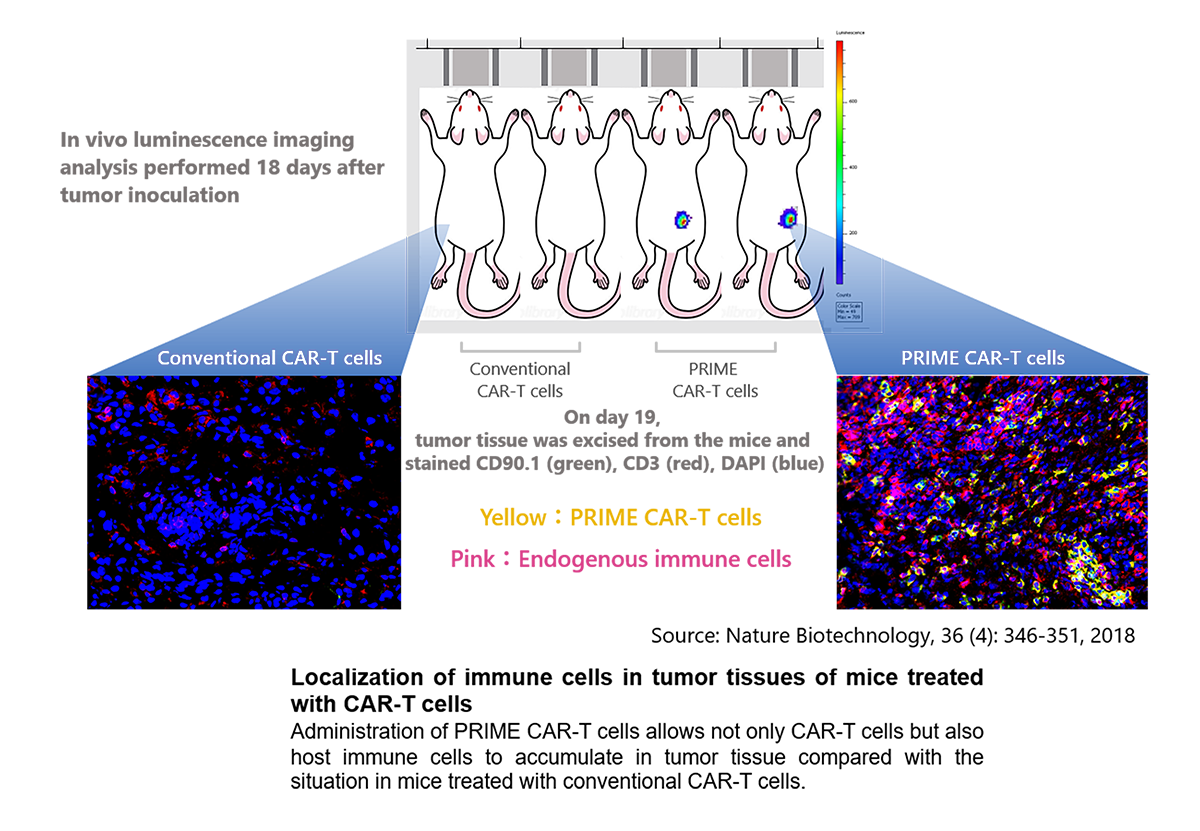
Moreover, in mice that once rejected a solid tumor after treatment with the PRIME CAR-T cell method, long-term prevention of recurrence was demonstrated not only against the CAR target-positive tumor cells, but also against the parental tumor cells lacking the CAR target antigen (see the above publication).
List of references related to PRIME technology
- Nature Biotechnology,36(4):346-351, 2018
- Cancer Immunol Immunother 70, 2503-2515, 2021
- Mol Cancer Ther. 21(1):138-148, 2022
Please check the video for an overview of PRIME CAR-T cell therapy
Noile-Immune
